| Overview |
|---|
Active since 2014, PHUSE’s Data Transparency Working Group provides subject matter expertise for the review of draft deliverables and guidance documents from regulatory bodies (such as the EMA and Health Canada), as well as other industry organisations (such as TransCelerate), and academia. Since their inception in 2020, the free-to-attend Data Transparency Events have gone from strength-to-strength. These virtual events have created an unrestrictive space where questions can be asked and challenges addressed. Individuals passionate about the area can come together to share vital knowledge, develop new ideas and spark innovation through presentations, panel discussions and Q&A sessions, alongside experts in the data sharing field. The PHUSE Data Transparency Winter Event will take place from 6–8 February 2024. Data Transparency Events offer you the chance to gain knowledge and experience from a wide data transparency community, allowing you to come together with expects from a variety of companies and backgrounds. During this virtual event, presentations will be delivered across the three days in bitesize chunks from 15:00-17:30 (GMT). There will also be a panel discussion and Q&A session focused on the day's themes. |
| Tuesday 6 February 15:00-17:30 (GMT) |
|---|
| A Cross-Sectional Study to Evaluate the Real-World Impact of Clinical Trial Transparency Initiatives, Shalini Dwivedi, Krystelis |
| Improving Data Findability Through Better Clinical Metadata, Lukasz Kniola, Biogen |
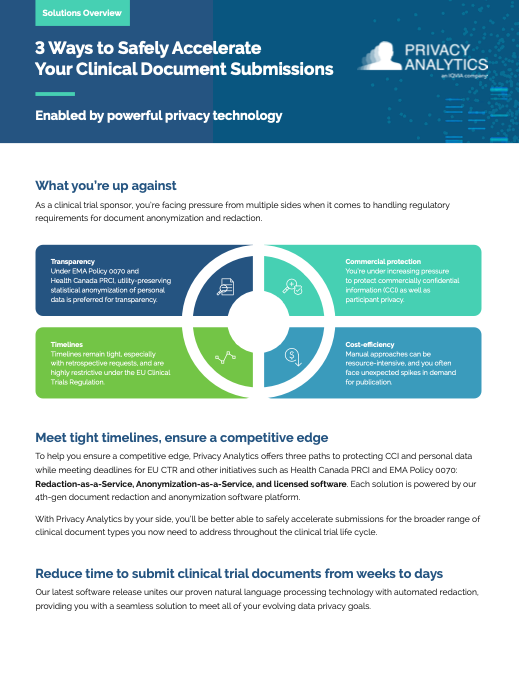
| Good Transparency Practices: A Working Group Update, Abby McDonell, Privacy Analytics and Lauren Hepburn, Rare Disease Sponsor |
| Lessons Learned in Anonymising Complex Health Data – Maximising Data Utility While Minimising Risk, Nuria Mackes, xValue and Asad Preuss-Dodhy, Roche Diagnostics |
| Wednesday 7 February 15:00-17:30 (GMT) |
| De-Identification Beyond Borders: Global Applicability of HIPAA Safe Harbor, Obaraboye Olude, Privacy Analytics |
| The HEAL Data Ecosystem: Enabling Data Sharing within the NIH HEAL Initiative®, Zixin Nie, RTI International |
| PSURs Public Release – Individual Patient Safety Data Disclosure Across Multiple Transparency Initiatives, Agnieszka Glowinska and Magdalena Majewska, AstraZeneca |
| The Final Countdown: Preparing for the 2025 CTIS Transition Deadline, Francine Lane, Citeline |
| Thursday 8 February 15:00-17:30 (GMT) |
| From Automated to Accountable: Building Responsible AI for Trial Transparency, Woo Song, Xogene |
| Balancing Act: The Dynamics of Legislation, AI, and Global Health Data Sharing, Luk Arbuckle, Privacy AnalyticsFrom Automated to Accountable: Building Responsible AI for Trial Transparency, Woo Song, Xogene |
| Challenges and Solutions to Anonymisation of Imaging Data (DICOM), Diwakar Angra, GENINVO |
| A Review of Biopharma Sponsor Data Sharing Policies and Protection Methodologies – Results from the 2023 Survey, Aaron Mann, Clinical Research Data Sharing Alliance & Luk Arbuckle, Privacy Analytics |
| Data Transparency Winter Event Sponsors |
|---|
Virtual Event Sponsors |
| Sponsor Flyers |
| Sponsorship |
Hosting the Data Transparency Events digitally means that no matter where you are in the world you can participate. It provides the industry with a broader opportunity to share knowledge on a global scale, connecting through the virtual event platform. The sponsor options offer a range of benefits with ample company exposure. See the prospectusfor more detail. |
| Data Transparency Working Group Leads |
|---|