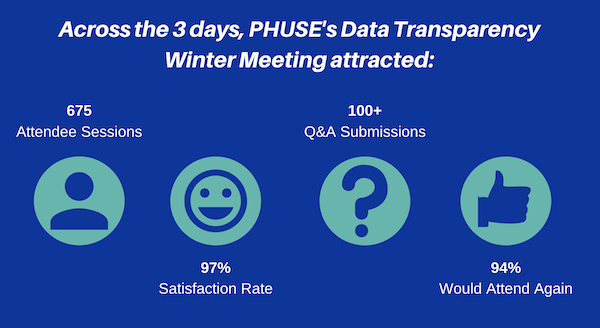
The field of data transparency within clinical development is fast-moving. PHUSE recognised the need to share information, advice and suggestions across all pharma companies (big and small), vendors and software companies, in a way that would not be cost-prohibitive – and so, the PHUSE Data Transparency Multi-Day Meeting was created.
Our first event was the Summer Meeting, back in June 2020. We have just held our second Meeting – the Data Transparency Winter Meeting (19th–21st January). This event ran for 2.5 hours each day from 15:00 to 17:30 (GMT). Each day explored a key topic: New Challenges for Data Sharing, Regulatory Submissions, and Explore Technologies. Attendees had the opportunity to listen to three/four live presentations, and then participate in a joint panel discussion/Q&A session based on the content from the day.
The event saw a variety of innovative presentations including:
- Data sharing of genetic data and rare diseases
- Experiences with regulatory submissions and insights into the MHRA Data Transparency approach
- Use of synthetic data to support under-recruiting studies
- Programming languages to implement data de-identification
- Data disclosure strategy
- Use of Plain Language Summary to support patient engagement
Thank you to our DT Winter Meeting 2021 sponsors...
A big thank you to all of our sponsors of the DT Winter Meeting 2021. This virtual event allowed us to screen our sponsors’ promotional videos in between presentation intervals, which was well received by our attendees.
Click below to view sponsor videos from our Platform Sponsor Privacy Analytics, and our Media Contributors Kinapse and d-wise, to find out more about what these companies do.