Image Removed
Image Removed Image Added
Image Added
Project Scope |
- Define role of QTLs in QbD, in particular the relationship to CTQs (Critical to Quality Factors), Estimands and continuous quality improvement (CQI)
- Discuss the use of QTLs in Early Development/small studies, bio equivalence and complex designs
- Discuss examples of how to define QTLs, different methodologies and different parameters in use across the industry
- Discuss difficulties and challenges of implementation of QTLs
- Examine role of QTLs as part of the of the lessons learned (RCA) feedback loop
|
| Andy Lawton | w.a.lawton@aol.co.uk |
Wendy Dobson (PHUSE Project Manager) | wendy@phuseProject MembersOrganisation| Adam Czernik | Janssen Research and Development |
| Andrew McGowan | RHO World |
| Andrzej Kinasiewicz | AstraZeneca |
| Anne Lawrence | AbbVie |
| Crupa Kurien | Pfizer |
| Julie Appel | Novo Nordisk |
| Kate Tomlinson | PRISM |
| Mary Arnould | Astellas |
| Project Members | Organisation |
|---|
| Michael Walega | BMS |
| Mireille Lovejoy | Roche |
| Monika Moersch | Boehringer-Ingelheim |
| Mukesh Babu | Industry |
| Paul Brown | Danish Medicines Agency |
| Priti Gupta | Industry |
| Sheetal Chandarana | Roche |
| Steve Young | CluePoints |
| Steven Gilbert | Pfizer |
|
|---|
- Continuing to work on their White Paper
|
Sukalpo Saha | TCS/Roche
| Objectives & Deliverables | Timelines |
| Publications | Q42021 |
| White Paper | Q22022 |
| Status |
|---|
| colour | Blue |
|---|
| title | Current Status |
|---|
|
Q22021 |
This is a new project and is actively seeking participation. If you are interested in joining this team, please email workinggroups@phuse.global.| Release of draft White Paper | Q3 2022 |
| Publish White Paper | Q3 2023 |
Problem Statement QTLs - the role they play in defining quality within the QbD framework, their relationship to Critical to Quality factors, associated methodologies and the interpretation of them have not been fully defined in clinical development, in particular where early development/small studies, bio equivalence studies and complex designs are concerned. Problem Impact This will impact the whole clinical development process and allow the move away from perfection to a defined and achievable quality, from which continuous quality improvement can begin. |
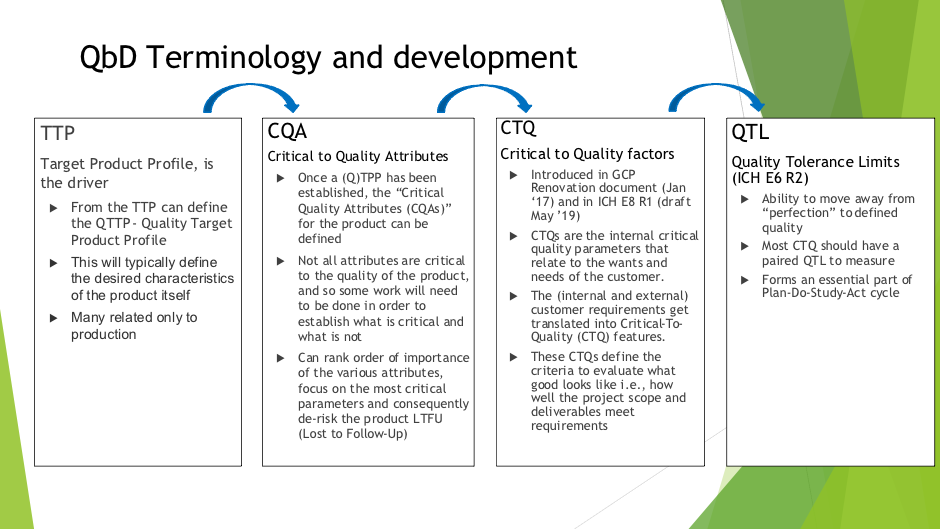
| Terminology Example: - Target Product Profile (TPP)
- Patient wants extended dosing from current BD product on market
- Critical Quality Attribute (CQA)
- Critical to Quality Factor (CTQ)
- In early stage trials drug levels shown at 24 hours
- In Phase 2-3 demonstrate efficacy
- Quality Tolerance Limit
- Pre-define what will be acceptable limits for acceptance
- These QTL's will change at different stages
|