Project Scope
|
| Project Leads | |
| Andy Lawton | w.a.lawton@aol.co.uk |
| Chris Wells | chris.wells.cw1@roche.com |
| Lauren White (PHUSE Project Co-ordinator) |
| Objectives & Deliverables | Timelines |
| Publications | Q42021 |
| White Paper | Q22022 |
CURRENT STATUS Q22021 |
This is a new project and is actively seeking participation. If you are interested in joining this team, please email workinggroups@phuse.global. Presentation sent to the FDA regarding the Project Scope, resulting in FDA members attending meetings when possible. - Q&A log. - Questionnaire in draft. |
| Project Members | Organisation |
| Adam Czernik | Janssen Research and Development |
| Alicja Mark | Genmab |
| Andrew McGowan | RHO World |
| Andrzej Kinasiewicz | AstraZeneca |
| Anne Lawrence | AbbVie |
| Ansalan Stewart | FDA |
| Arati Todkar | TCS |
| Chonna Campbell | Unither Pharmaceuticals |
| Crupa Kurien | Pfizer |
| Georgina Wood | Cyntegrity |
| Heather Turner | Prism |
| Heidi Hoffman | Genentech |
| Jean Mulinde | FDA |
| Julie Appel | Novo Nordisk |
| Karen Bleich | FDA |
| Kate Tomlinson | PRISM |
| Linda Del Paggio | Genentech |
| Lukasz Bojarski | AstraZeneca |
| Mary Arnould | Astellas |
| Project Members | Organisation |
| Michael Walega | BMS |
| Mireille Lovejoy | Roche |
| Monika Moersch | Boehringer-Ingelheim |
| Mukesh Babu | Industry |
| Nick Wells | Syneos Health |
| Paul Brown | Danish Medicines Agency |
| Priti Gupta | Pfizer |
| Shalaka Gadhave | TCS |
| Sheetal Chandarana | Roche |
| Steve Young | CluePoints |
| Steven Gilbert | Pfizer |
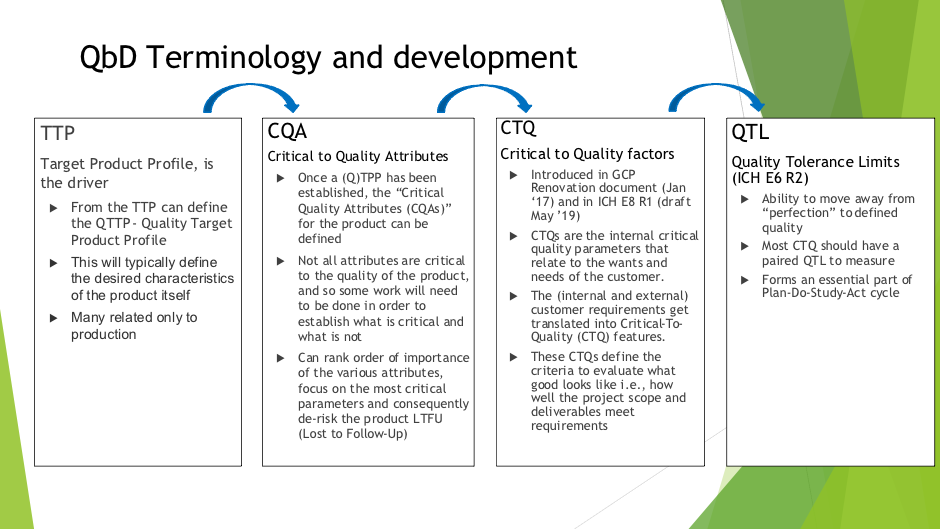
Problem Statement QTLs - the role they play in defining quality within the QbD framework, their relationship to Critical to Quality factors, associated methodologies and the interpretation of them have not been fully defined in clinical development, in particular where early development/small studies, bio equivalence studies and complex designs are concerned. Problem Impact This will impact the whole clinical development process and allow the move away from perfection to a defined and achievable quality, from which continuous quality improvement can begin. |
Terminology Example:
|