Image Removed
Image Removed Image Added
Image Added
Project Scope |
- Define role of QTLs in QbD, in particular the relationship to CTQs (Critical to Quality Factors), Estimands and continuous quality improvement (CQI)
- Discuss the use of QTLs in Early Development/small studies, bio equivalence and complex designs
- Discuss examples of how to define QTLs, different methodologies and different parameters in use across the industry
- Discuss difficulties and challenges of implementation of QTLs
- Examine role of QTLs as part of the of the lessons learned (RCA) feedback loop
|
w.a.lawton@aol.co.uk | Lauren White (| Alex Pearce, PHUSE Project |
Lauren@phuse.global | | Objectives & Deliverables | Timelines |
| Publications | Q42021 |
White Paper | Q22022
| Status |
|---|
| colour | Blue |
|---|
| title | Current Status |
|---|
|
|
|---|
This is a new project and is actively seeking participation. If you are interested in joining this team, please email workinggroups@phuse.global. Presentation sent to the FDA regarding the Project Scope, resulting in FDA members attending meetings when possible. - Q&A log. - Questionnaire in draft. |
|
|---|
- Continuing to work on their White Paper
|
| Objectives & Deliverables | Timelines |
| Release of draft White Paper | Q3 2022 |
| Publish White Paper | Q3 2023 |
| Project Members | Organisation |
| Adam Czernik | Janssen Research and Development |
| Alicja Mark | Genmab |
| Andrew McGowan | RHO World |
| Andrzej Kinasiewicz | AstraZeneca |
| Anne Lawrence | AbbVie |
| Ansalan Stewart | FDA |
| Arati Todkar | TCS |
| Chonna Campbell | Unither Pharmaceuticals |
| Crupa Kurien | Pfizer |
| Georgina Wood | Cyntegrity |
| Heather Turner | Prism |
| Heidi Hoffman | Genentech |
| Jean Mulinde | FDA |
| Julie Appel | Novo Nordisk |
| Karen Bleich | FDA |
| Kate Tomlinson | PRISM |
| Linda Del Paggio | Genentech |
| Lukasz Bojarski | AstraZeneca |
| Mary Arnould | Astellas |
| Project Members | Organisation |
| Michael Walega | BMS |
| Mireille Lovejoy | Roche |
| Monika Moersch | Boehringer-Ingelheim |
| Mukesh Babu | Industry |
| Nick Wells | Syneos Health |
| Paul Brown | Danish Medicines Agency |
| Priti Gupta | Pfizer |
| Shalaka Gadhave | TCS |
| Sheetal Chandarana | Roche |
| Steve Young | CluePoints |
Steven Gilbert | Pfizer
Problem Statement QTLs - the role they play in defining quality within the QbD framework, their relationship to Critical to Quality factors, associated methodologies and the interpretation of them have not been fully defined in clinical development, in particular where early development/small studies, bio equivalence studies and complex designs are concerned. Problem Impact This will impact the whole clinical development process and allow the move away from perfection to a defined and achievable quality, from which continuous quality improvement can begin. |
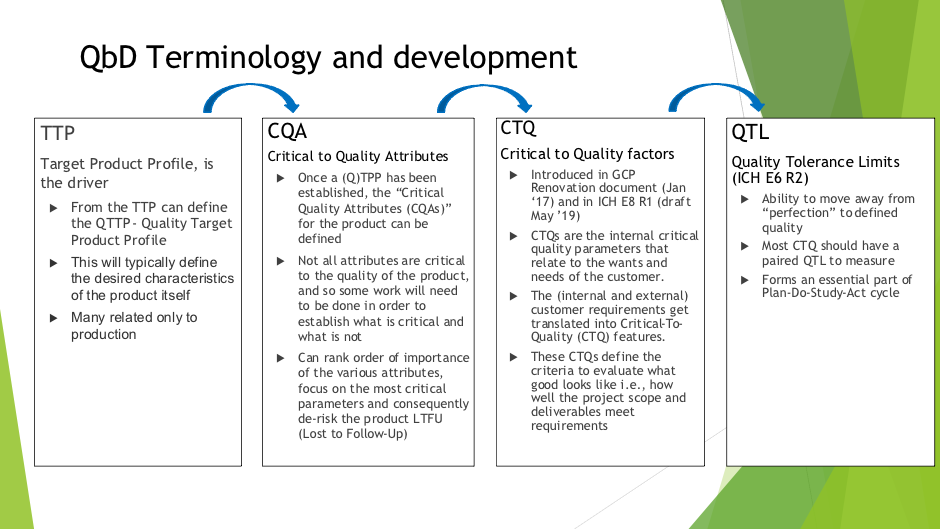
| Terminology Example: - Target Product Profile (TPP)
- Patient wants extended dosing from current BD product on market
- Critical Quality Attribute (CQA)
- Critical to Quality Factor (CTQ)
- In early stage trials drug levels shown at 24 hours
- In Phase 2-3 demonstrate efficacy
- Quality Tolerance Limit
- Pre-define what will be acceptable limits for acceptance
- These QTL's will change at different stages
|